kinesin
Conformational Selection & Allostery in Molecular Switches
29/09/14 14:53
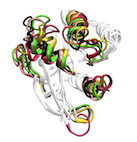
Our atomistic molecular simulations indicate that these enzymes harbor an intrinsic susceptibility to sample multiple conformational states regardless of the bound nucleotide. These results indicate that conformational changes in these enzymes may be best described by a population-shift mechanism rather than by the popular ligand induced-fit on/off switch model. Read More...
Molecular Motors & the Cytoskeleton
29/09/14 14:53
We are fascinated by molecular motion, particularly that of nature’s motor proteins: kinesin, myosin and dynein. Together with their cellular highways, microtubules and actin filaments, these proteins drive the beating of sperm, the division of cells and the muscular movement of organisms. Our research is focused on deciphering how these mighty molecular motors work, and how to manipulate them for industrial and medical advantage.
A principal aim in the study of molecular motors, is a detailed understanding of how chemical energy, derived from the hydrolysis of ATP, is converted into mechanical energy. Read More...
